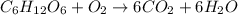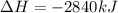Chemistry, 01.08.2019 16:30 carlalopezelox2244
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), according to the equation below. mc020-1.jpg the enthalpy of the reaction is –2,840 kj. what is the heat of combustion, per mole, of glucose? –2,840 kj/mol –473.3 kj/mol 473.3 kj/mol 2,840 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), acco...
Questions






Mathematics, 07.01.2021 16:50


Mathematics, 07.01.2021 16:50





Mathematics, 07.01.2021 16:50

Health, 07.01.2021 16:50

English, 07.01.2021 16:50



Mathematics, 07.01.2021 16:50


Biology, 07.01.2021 16:50