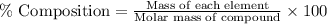Chemistry, 01.08.2019 23:30 jennifercastill3
15. a 22.4-l sample of which of the following substances, at stp, would contain 6.02 x 10^23 representative particles? *a. goldb. sodium chloridec. sulfurd. carbon dioxide16. what information is needed to calculate the percent composition of a compound? *a. the formula of the compound and the atomic mass of its elementsb. the formula of the compound and its volumec. the weight of the sample to be analyzed and its molar volumed. the weight of the sample to be analyzed and its state of matter

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Cobalt-60 is an artificial radioisotope that is produced in a nuclear reactor and is used as a gamma-ray source in the treatment of certain types of cancer. if the wavelength of the gamma radiation from a cobalt-60 source is 1.00 × 10-3 nm, calculate the energy of a photon of this radiation.
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
15. a 22.4-l sample of which of the following substances, at stp, would contain 6.02 x 10^23 represe...
Questions


Advanced Placement (AP), 17.01.2022 08:50

History, 17.01.2022 08:50

Mathematics, 17.01.2022 08:50


English, 17.01.2022 09:00

Mathematics, 17.01.2022 09:00

Mathematics, 17.01.2022 09:00




English, 17.01.2022 09:00

Mathematics, 17.01.2022 09:00

English, 17.01.2022 09:00

World Languages, 17.01.2022 09:00

Mathematics, 17.01.2022 09:00

Business, 17.01.2022 09:00


English, 17.01.2022 09:00

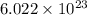 number of particles.
number of particles.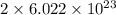 number of particles.
number of particles.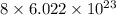 number of particles.
number of particles.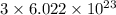 number of particles.
number of particles.