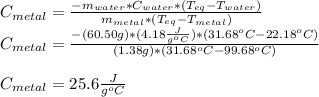Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
Astudent places 1.38 g of unknown metal at 99.68c into 60.50 g of water at 22.18c. the entire system...
Questions

Mathematics, 01.09.2021 14:00

Mathematics, 01.09.2021 14:00

English, 01.09.2021 14:00

Mathematics, 01.09.2021 14:00

Mathematics, 01.09.2021 14:00




English, 01.09.2021 14:00


Physics, 01.09.2021 14:00

Biology, 01.09.2021 14:00

Social Studies, 01.09.2021 14:00



Mathematics, 01.09.2021 14:00


Mathematics, 01.09.2021 14:00

Mathematics, 01.09.2021 14:00


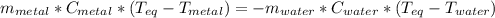
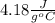 and the given data, one obtains:
and the given data, one obtains: