
Chemistry, 02.08.2019 02:00 daydallas01
Carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond covalently, which of the following is the correct lewis dot (electron dot) structure for carbon dioxide?
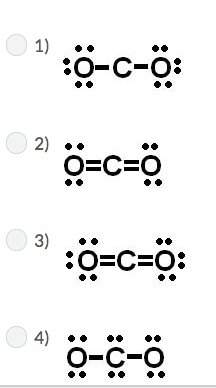

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1


Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
You know the right answer?
Carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond c...
Questions



Mathematics, 20.09.2020 15:01

Mathematics, 20.09.2020 15:01

English, 20.09.2020 15:01

Mathematics, 20.09.2020 15:01


Biology, 20.09.2020 15:01





Mathematics, 20.09.2020 15:01



Chemistry, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01


English, 20.09.2020 15:01



