Chemistry, 02.08.2019 02:30 imstupid77
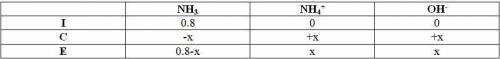
Ammonia, nh3, is a weak base with a kb = 1.8 x 10-5. in a 0.8m solution of ammonia, which has a higher concentration: ammonia (nh3) or its conjugate acid the ammonium ion (nh4+)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Ammonia, nh3, is a weak base with a kb = 1.8 x 10-5. in a 0.8m solution of ammonia, which has a high...
Questions





Geography, 17.03.2020 23:25

Mathematics, 17.03.2020 23:25

Mathematics, 17.03.2020 23:25

Mathematics, 17.03.2020 23:25

Mathematics, 17.03.2020 23:25

Mathematics, 17.03.2020 23:25

Law, 17.03.2020 23:25

Mathematics, 17.03.2020 23:25






Business, 17.03.2020 23:25