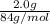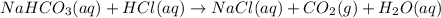If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon dioxide should one tablet yield? compare this theoretical value with your results. (hint: you will first need to convert your mass into moles by dividing by the molar mass of nahco3 or sodium hydrogen carbonate a/k/a baking soda). , show all work and think about mole ratios relating co2 to nahco3.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

You know the right answer?
If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon di...
Questions



Mathematics, 08.11.2019 09:31



History, 08.11.2019 09:31

Mathematics, 08.11.2019 09:31


Mathematics, 08.11.2019 09:31




Mathematics, 08.11.2019 09:31

Business, 08.11.2019 09:31



History, 08.11.2019 09:31

Biology, 08.11.2019 09:31

Biology, 08.11.2019 09:31

Social Studies, 08.11.2019 09:31

 (1)
(1)