
Chemistry, 13.01.2020 23:31 smelcher3900
Given the reaction system in a closed container at equilibrium and at a temperature of 298 k:
n2o4(g) < ==> 2no2(g)
the measurable quantities of the gases at equilibrium must be
(1) decreasing (3) equal
(2) increasing (4) constant

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1


Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Given the reaction system in a closed container at equilibrium and at a temperature of 298 k:
...
...
Questions

Mathematics, 24.06.2021 06:30

Mathematics, 24.06.2021 06:30

Mathematics, 24.06.2021 06:30

Mathematics, 24.06.2021 06:30



English, 24.06.2021 06:40


Mathematics, 24.06.2021 06:40

Mathematics, 24.06.2021 06:40

Physics, 24.06.2021 06:40



Mathematics, 24.06.2021 06:40

Mathematics, 24.06.2021 06:40

Business, 24.06.2021 06:40

Mathematics, 24.06.2021 06:40

Mathematics, 24.06.2021 06:40


Social Studies, 24.06.2021 06:40

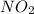 , rate of formation of
, rate of formation of 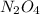 , pressure of
, pressure of 

