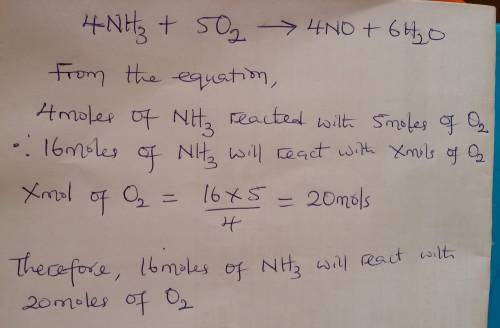Given the balanced equation representing a reaction:
4nh3 + 5o2 ==> 4no + 6h2o
what...

Chemistry, 17.11.2019 07:31 michelle5642b
Given the balanced equation representing a reaction:
4nh3 + 5o2 ==> 4no + 6h2o
what is the minimum number of moles of o2 that are needed to completely react with 16 moles of nh3?
(1) 16 mol (3) 64 mol
(2) 20. mol (4) 80. mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Questions

Mathematics, 26.10.2020 23:20

History, 26.10.2020 23:20



English, 26.10.2020 23:20

History, 26.10.2020 23:20


Computers and Technology, 26.10.2020 23:20


Mathematics, 26.10.2020 23:20

Mathematics, 26.10.2020 23:20

Mathematics, 26.10.2020 23:20

English, 26.10.2020 23:20

History, 26.10.2020 23:20



Mathematics, 26.10.2020 23:20


Biology, 26.10.2020 23:20