Given the reaction:
ch4(g) + 2 o2(g) --> 2 h2o(g) + co2(g)
what is the overall resul...

Chemistry, 06.10.2019 01:30 hayleylatti8691
Given the reaction:
ch4(g) + 2 o2(g) --> 2 h2o(g) + co2(g)
what is the overall result when ch4(g) burns
according to this reaction?
(1) energy is absorbed and dh is negative.
(2) energy is absorbed and dh is positive.
(3) energy is released and dh is negative.
(4) energy is released and dh is positive.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1


You know the right answer?
Questions


Business, 04.12.2020 21:30


English, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30


Biology, 04.12.2020 21:30



Health, 04.12.2020 21:30


Mathematics, 04.12.2020 21:30

Biology, 04.12.2020 21:30

Mathematics, 04.12.2020 21:30



Computers and Technology, 04.12.2020 21:30


Mathematics, 04.12.2020 21:30

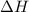 is negative.
is negative.