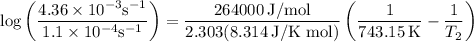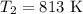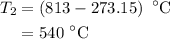Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
The rate constant of a reaction is 1.1 × 10-4 s-1 at 470 °c, and the activation energy is 264 kj/mol...
Questions

Mathematics, 20.09.2021 15:50

Mathematics, 20.09.2021 15:50


English, 20.09.2021 15:50


Mathematics, 20.09.2021 15:50

English, 20.09.2021 15:50

History, 20.09.2021 15:50



Mathematics, 20.09.2021 15:50




English, 20.09.2021 15:50

Mathematics, 20.09.2021 15:50

Mathematics, 20.09.2021 15:50



Mathematics, 20.09.2021 15:50

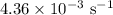 comes out to be
comes out to be  .
.
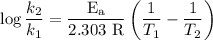 …… (1)
…… (1)
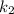 is rate constant at temperature
is rate constant at temperature 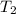 .
.
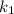 is rate constant temperature
is rate constant temperature 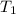 .
.
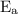 is activation energy.
is activation energy.
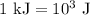
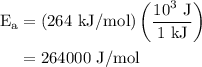
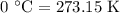
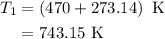
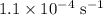 .
.