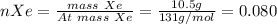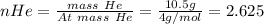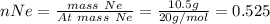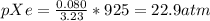Chemistry, 03.08.2019 03:30 pinapunapula
In a mixture of he, ne, and xe gases with a total pressure of 925 atm, if there is 10.5 g of each gas in the mixture, what is the partial pressure of xe?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
In a mixture of he, ne, and xe gases with a total pressure of 925 atm, if there is 10.5 g of each ga...
Questions


Mathematics, 22.04.2021 08:10




English, 22.04.2021 08:10

Medicine, 22.04.2021 08:10

History, 22.04.2021 08:10

World Languages, 22.04.2021 08:10

Mathematics, 22.04.2021 08:10




Social Studies, 22.04.2021 08:10

Social Studies, 22.04.2021 08:10





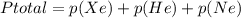 -------------(1)
-------------(1)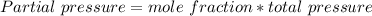
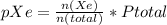 -----(2)
-----(2)