
Chemistry, 03.08.2019 05:30 krystlemiller4307
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 . if the molar mass of the compound is 129.1 g/mol, what is the chemical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 ....
Questions

History, 01.02.2020 00:55


Biology, 01.02.2020 00:55



History, 01.02.2020 00:55


Mathematics, 01.02.2020 00:55




English, 01.02.2020 00:55


English, 01.02.2020 00:55





History, 01.02.2020 00:55

Mathematics, 01.02.2020 00:55

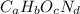 . The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
. The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.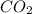 , 1.09 g of
, 1.09 g of 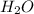 and 1.70 g of
and 1.70 g of 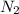 . First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
. First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.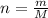
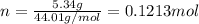
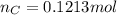
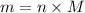
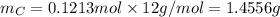
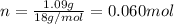
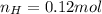
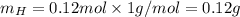
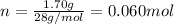
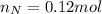
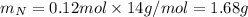
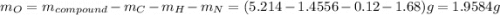
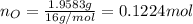
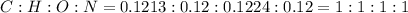
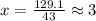
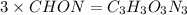
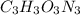 .
.

