
Chemistry, 30.10.2019 21:31 demetriascott20
According to the collision theory of chemical reactions, an increase in the number of effective reactant collisions per unit time will increase the rate of the reaction.
true
false
you can study reaction rates to answer which of the following questions?
will the reaction go to completion?
will the reaction occur?
how can the reaction be slowed down?
how many grams of product will be made?
changing the concentration of one or more of reactants can affect the rate of a reaction.
true
false
if the surface area of reactants decreases:
the reaction rate is generally lower.
the reaction rate is generally higher.
the reaction rate is not affected.
the effect on reaction rate cannot be predicted.
which of the following would increase the rate of a chemical reaction?
reducing the temperature
increasing volume
using reactants in fine, powder form, rather than large, chunky form
decreasing reactant concentration
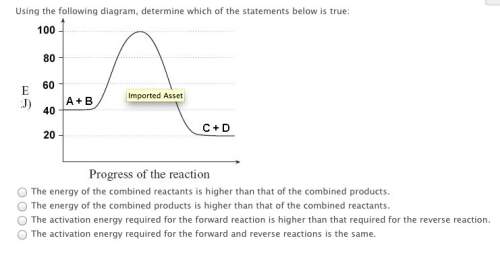
in an energy diagram for an endothermic reaction, the products are higher in energy than the reactants.
true
false
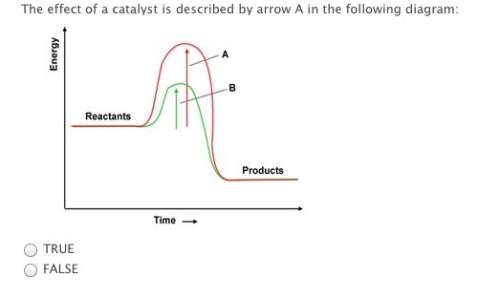
a catalyst works by increasing the activation energy needed for a reaction.
true
false


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1


Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
According to the collision theory of chemical reactions, an increase in the number of effective reac...
Questions



Biology, 28.01.2020 02:31



Mathematics, 28.01.2020 02:31


Mathematics, 28.01.2020 02:31

Mathematics, 28.01.2020 02:31




History, 28.01.2020 02:31


Chemistry, 28.01.2020 02:31

History, 28.01.2020 02:31



Chemistry, 28.01.2020 02:31



