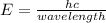Chemistry, 03.08.2019 17:30 rachelreed
When an electron of an atom falls from a higher energy level to the ground state, the atom loses 9.4145 x 10-25 joules of energy. what is the wavelength of the radiation emitted as a result of this transition? (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108m/s)?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
When an electron of an atom falls from a higher energy level to the ground state, the atom loses 9.4...
Questions

Mathematics, 13.08.2021 09:00

English, 13.08.2021 09:00

History, 13.08.2021 09:00



Mathematics, 13.08.2021 09:00


Mathematics, 13.08.2021 09:00



Social Studies, 13.08.2021 09:00

Health, 13.08.2021 09:00




Mathematics, 13.08.2021 09:00


Computers and Technology, 13.08.2021 09:00

English, 13.08.2021 09:00

History, 13.08.2021 09:00