
Chemistry, 03.08.2019 18:30 prettygirllniyiaa
Gef3h is formed from geh4 and gef4 in the combination reaction: geh4 + 3gef4 -> 4gef3h. if the reactiln yield is 92.6%, how many moles of gef4 are needed to produce 8 mol of gef3h?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3


Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Gef3h is formed from geh4 and gef4 in the combination reaction: geh4 + 3gef4 -> 4gef3h. if the...
Questions


English, 13.02.2021 21:10



Biology, 13.02.2021 21:10




Spanish, 13.02.2021 21:10


Arts, 13.02.2021 21:10

Mathematics, 13.02.2021 21:10

Mathematics, 13.02.2021 21:10


Mathematics, 13.02.2021 21:10




Mathematics, 13.02.2021 21:10

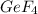 needed to produce 8 mole of
needed to produce 8 mole of 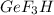 are, 6.48 moles.
are, 6.48 moles.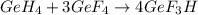
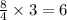 mole of
mole of 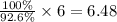 moles of
moles of 

