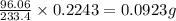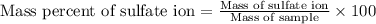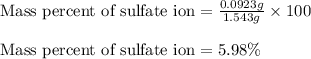Chemistry, 03.08.2019 22:30 sindy35111
A1.543 gram sample containing sulfate ion was treated with barium chloride reagent, and 0.2243 grams of barium sulfate was isolated. calculate the percentage of sulfate ion in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1
You know the right answer?
A1.543 gram sample containing sulfate ion was treated with barium chloride reagent, and 0.2243 grams...
Questions

Social Studies, 22.09.2019 03:30


History, 22.09.2019 03:30



English, 22.09.2019 03:30

Mathematics, 22.09.2019 03:30

Chemistry, 22.09.2019 03:30




Health, 22.09.2019 03:30

Mathematics, 22.09.2019 03:30