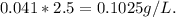Chemistry, 03.08.2019 22:30 josephvaldez518
The solubility of oxygen gas in water at 25 °c and 1.0 atm pressure of oxygen is 0.041 g/l. the solubility of oxygen in water at 2.5 atm and 25 °c is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
The solubility of oxygen gas in water at 25 °c and 1.0 atm pressure of oxygen is 0.041 g/l. the solu...
Questions


Business, 16.11.2020 18:10


Arts, 16.11.2020 18:10








Mathematics, 16.11.2020 18:10