
Chemistry, 04.08.2019 00:30 bullockarwen
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) → 2mgo(s) how many moles of o2 are consumed when 0.550 mol of magnesium burns?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
You know the right answer?
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2mg(s) + o2(g) → 2mgo...
Questions

English, 25.01.2021 21:50

English, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50


Mathematics, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Chemistry, 25.01.2021 21:50





Biology, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50

Physics, 25.01.2021 21:50

English, 25.01.2021 21:50

Mathematics, 25.01.2021 21:50


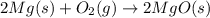
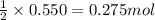 of oxygen gas.
of oxygen gas.

