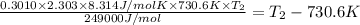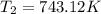Chemistry, 04.08.2019 02:00 natalieoppelt
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 hcl the activation energy is 249 kj/mol and the frequency factor is 1.6 × 1014 s−1. find the temperature at which the rate of the reaction would be twice as fast as when the reaction runs at 730.6 k. enter your answer numerically and in terms of kelvin.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

You know the right answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 hcl the activation energy...
Questions


Mathematics, 20.09.2019 15:30


Mathematics, 20.09.2019 15:30

Social Studies, 20.09.2019 15:30




Mathematics, 20.09.2019 15:30


History, 20.09.2019 15:30

History, 20.09.2019 15:30



Mathematics, 20.09.2019 15:30

Computers and Technology, 20.09.2019 15:30

Chemistry, 20.09.2019 15:30


History, 20.09.2019 15:30

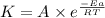
![\log \frac{K_2}{K_1}=\frac{E_a}{2.303R}\times [\frac{T_2-T_1}{T_1T_2}]](/tpl/images/0167/7789/e0c66.png)
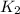 = rate of reaction at
= rate of reaction at 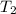
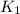 = rate of reaction at
= rate of reaction at 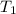
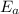 = activation energy
= activation energy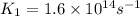
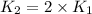
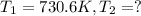
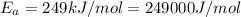
![\log \frac{2K_1}{K_1}=\frac{249000 kJ/mol}{2.303\times 8.314 J/mol K}\times [\frac{T_2-730.6 K}{730.6 K\times T_2}]](/tpl/images/0167/7789/638fd.png)
![0.3010=\frac{249000 J/mol}{2.303\times 8.314 J/mol K}\times [\frac{T_2-730.6 K}{730.6 K\times T_2}]](/tpl/images/0167/7789/3fa9a.png)