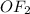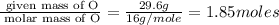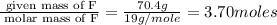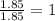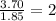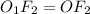Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1


Chemistry, 24.06.2019 01:30
Rita divides avogadro’s number (approximately 6.02214 x 10^23) by 2.055 to calculate the number of atoms in a sample. which expression gives her result to the correct number of significant figures?
Answers: 3

Chemistry, 24.06.2019 03:30
Give the formula of each coordination compound. include square brackets around the coordination complex. do not include the oxidation state on the metal. use parentheses only around polyatomic ligands. sodium (iii)
Answers: 1
You know the right answer?
What is the empirical formula of a compound consisting of 29.6% oxygen and 70.4% fluorine by mass?...
Questions


Mathematics, 30.10.2019 12:31





Spanish, 30.10.2019 12:31


Biology, 30.10.2019 12:31

Biology, 30.10.2019 12:31


Social Studies, 30.10.2019 12:31


Mathematics, 30.10.2019 12:31

Mathematics, 30.10.2019 12:31


Mathematics, 30.10.2019 12:31

Social Studies, 30.10.2019 12:31

Mathematics, 30.10.2019 12:31

Arts, 30.10.2019 12:31