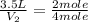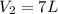The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(g). if a balloon was filled with 3.5l of ammonia gas and all of the gas decomposed, what would be the total volume of gas in the container? assume the pressure and temp remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(...
Questions

History, 24.12.2019 08:31

Biology, 24.12.2019 08:31

English, 24.12.2019 08:31


Geography, 24.12.2019 08:31


Social Studies, 24.12.2019 08:31

Business, 24.12.2019 08:31


History, 24.12.2019 08:31

History, 24.12.2019 08:31

History, 24.12.2019 08:31

Chemistry, 24.12.2019 08:31




Mathematics, 24.12.2019 08:31


Geography, 24.12.2019 08:31

English, 24.12.2019 08:31

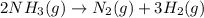

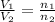
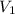 = initial volume of ammonia gas = 3.5 L
= initial volume of ammonia gas = 3.5 L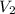 = total volume of gas in the container = ?
= total volume of gas in the container = ?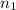 = initial moles of ammonia gas = 2 mole
= initial moles of ammonia gas = 2 mole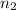 = final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles
= final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles