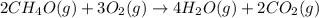Answers: 2


Another question on Chemistry




Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
The combustion of methanol (ch4o) is described by this balanced chemical equation: 2ch4o(g) + 3o2(g...
Questions




Mathematics, 22.06.2021 03:40


Mathematics, 22.06.2021 03:40


Mathematics, 22.06.2021 03:40

English, 22.06.2021 03:40

Mathematics, 22.06.2021 03:40


Physics, 22.06.2021 03:40




Computers and Technology, 22.06.2021 03:50


Mathematics, 22.06.2021 03:50

Business, 22.06.2021 03:50