
Chemistry, 04.08.2019 10:30 ambarpena14
Agas is sealed in a bottle at 25°c and 1.50 atm pressure. how must the storage conditions be changed to bring the gas to standard temperature and pressure (stp)? increase the temperature by 25°c and decrease the pressure by 0.514 atm. keep the temperature the same and increase the pressure by 98.5 atm. decrease the temperature by 25°c and decrease the pressure by 0.500 atm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Agas is sealed in a bottle at 25°c and 1.50 atm pressure. how must the storage conditions be changed...
Questions


English, 27.10.2020 01:00

Spanish, 27.10.2020 01:00





Mathematics, 27.10.2020 01:00


History, 27.10.2020 01:00



English, 27.10.2020 01:00

Chemistry, 27.10.2020 01:00


Biology, 27.10.2020 01:00


Mathematics, 27.10.2020 01:00

Spanish, 27.10.2020 01:00

Mathematics, 27.10.2020 01:00

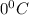 and an absolute pressure of exactly 1 atm.
and an absolute pressure of exactly 1 atm.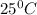 and 1.50 atmosphere to bring to STP , the temperature must be decreased by
and 1.50 atmosphere to bring to STP , the temperature must be decreased by 

