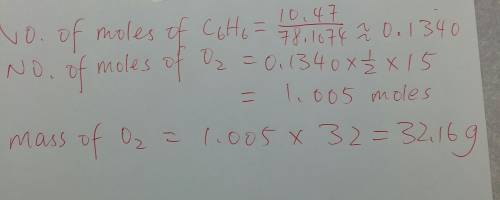Chemistry, 04.08.2019 13:00 67555savchenko
In the following reaction, how many grams of oxygen will react with 10.47 grams of benzene (c6h6)? 2c6h6 + 1502 12co2 + 6h2o the molar mass of benzene is 78.1074 grams and that of o2 is 32 grams.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
In the following reaction, how many grams of oxygen will react with 10.47 grams of benzene (c6h6)?...
Questions



Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01



Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Social Studies, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01



Mathematics, 03.08.2020 14:01


Computers and Technology, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01