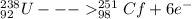proton produces nuclide x and a neutron. what is nuclide x?

Chemistry, 25.08.2019 01:30 lathwkuster
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
answers:
magnesium-24
magnesium-23
neon-23
s
odium-24
none of the above
the isotope p
has a half-life of 14.3 days. if a sample originally contained 1.00 g of p,
how much was left after 43 days?
answers:
0.250 g
0.125 g
0.750 g
0.500 g
identify x in the reaction
below.
u
+ c
→ cf
+ x
answers:
1
alpha particle
3
protons
6
neutrons
6
electrons
a .20 gram sample of c-14 was allowed to decay for 3 half-lives. what mass
of the sample will remain? carbon-14 has a half life of 5730
years.
answers:
0.025
0.05
0.1
0
.01
0.05
the isotope cu
has a half-life of 30 s. if a sample originally contained 48 mg of cu,
how much time passed before the amount fell to 3 mg?
answers:
120 s
240s
30 s
60 s(when i did my calculation for the question above, i got 60 seconds)
what radionuclide decays to fe-56
by beta emission?
answers:
fe
co
mn
co
mnhow do i know wat it becomes. i put 57 co 27 and it's wrong.
the cf
to cf
conversion is accompanied by
answers:
an alpha
emission
a
neutron capture
an electron
capture
an electron releasei would greatly appreciate your . you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
bombarding sodium-23 with a
proton produces nuclide x and a neutron. what is nuclide x?
proton produces nuclide x and a neutron. what is nuclide x?
Questions



Mathematics, 17.12.2020 21:10

Mathematics, 17.12.2020 21:10



Social Studies, 17.12.2020 21:10

Computers and Technology, 17.12.2020 21:10

History, 17.12.2020 21:10

Mathematics, 17.12.2020 21:10


Mathematics, 17.12.2020 21:10

Mathematics, 17.12.2020 21:10



English, 17.12.2020 21:10


English, 17.12.2020 21:10


History, 17.12.2020 21:10