
Chemistry, 04.08.2019 22:00 connermichaela
Aproposed mechanism for the oxidation of nitric oxide to nitrogen dioxide is shown below. 2 no (g) ⇄ n2o2 (g) fast, reversible step n2o2 (g) + o2 (g) → 2 no2 (g) slow step what rate law is consistent with this mechanism?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Aproposed mechanism for the oxidation of nitric oxide to nitrogen dioxide is shown below. 2 no (g) ⇄...
Questions


Mathematics, 06.11.2020 02:20

Mathematics, 06.11.2020 02:20

Mathematics, 06.11.2020 02:20




Chemistry, 06.11.2020 02:20

Geography, 06.11.2020 02:20

Spanish, 06.11.2020 02:20

Arts, 06.11.2020 02:20


Biology, 06.11.2020 02:20




Mathematics, 06.11.2020 02:20


Computers and Technology, 06.11.2020 02:20

![k_2[N_2O_2][O_2]](/tpl/images/0170/9744/230a2.png)
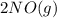 ⇄
⇄ 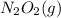 fast, reversible step
fast, reversible step 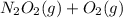 →
→ 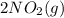
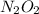 is NOT a reactant or a product. So then we should eliminate it from the rate law.
is NOT a reactant or a product. So then we should eliminate it from the rate law.![k_f [NO]^2 = k_r [N_2O_2]](/tpl/images/0170/9744/b35f7.png)
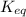 is:
is:
![\frac{k_f}{k_e} = \frac{[N_2O_2]}{[NO]^2} =K_{eq}](/tpl/images/0170/9744/80e33.png)
![[N_2O_2] = \frac{k_f}{k_r} [NO]^2](/tpl/images/0170/9744/c0de9.png)
![[N_2O_2] = \frac{k_f}{k_r}[NO]^2](/tpl/images/0170/9744/dec53.png)
![k_2 [N_2O_2][O_2] = k_2 \frac{k_f}{k_r} [NO]^2[O_2]](/tpl/images/0170/9744/cb3bb.png)
![k_{observed} [NO]^2[O_2] = k_2 K_{eq}[NO]^2[O_2]](/tpl/images/0170/9744/813cc.png)


