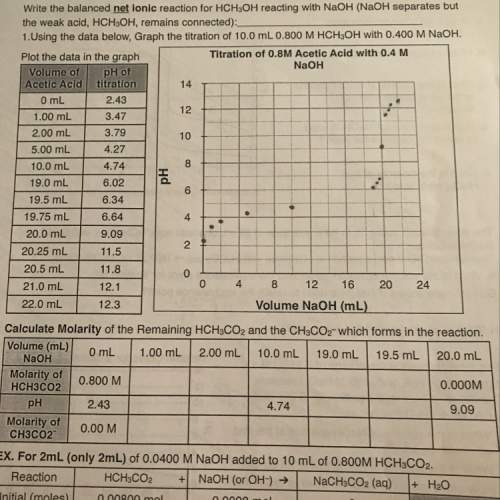
Seriously don't understand this worksheet at all !
...

Chemistry, 21.09.2019 21:30 lyd1aangela
Seriously don't understand this worksheet at all !

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1



Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Questions

Mathematics, 12.10.2020 20:01


Physics, 12.10.2020 20:01



Spanish, 12.10.2020 20:01



Physics, 12.10.2020 20:01


Arts, 12.10.2020 20:01

Social Studies, 12.10.2020 20:01


Physics, 12.10.2020 20:01

Law, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01

Advanced Placement (AP), 12.10.2020 20:01


Mathematics, 12.10.2020 20:01



