
Chemistry, 04.08.2019 22:30 kyliemorgan8623
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg ab (fast) and the rate = k[a][b] step 2: ab + b mc016-2.jpg ab2 (slow) and the rate = k[ab][b] step 3: ab2 + b mc016-3.jpg ab3 (fast) and the rate = k[ab2][b] overall: a + 3b mc016-4.jpg ab3 and the rate = k[a][b]2 which explains why the rate law for the overall equation is not the same as the rate equation for the rate-determining step?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg...
Questions


Chemistry, 25.07.2019 07:00



Biology, 25.07.2019 07:00

History, 25.07.2019 07:00







Health, 25.07.2019 07:00

History, 25.07.2019 07:00

Biology, 25.07.2019 07:00





![A+B\rightleftharpoons AB(fast)\\Rate=K[A][B]](/tpl/images/0171/0396/8b588.png)
![AB+B\rightarrow AB_2(slow)\\Rate=K[AB][B]](/tpl/images/0171/0396/fb2ef.png)
![AB_2+B\rightleftharpoons AB_3(fast)\\Rate=K[AB_2][B]](/tpl/images/0171/0396/ac6cb.png)
![Rate=K[A][B]^2](/tpl/images/0171/0396/92d0b.png)
![Rate=K[AB][B]](/tpl/images/0171/0396/058ce.png) ...........(A)
...........(A)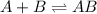
![K=\frac{[AB]}{[A][B]}](/tpl/images/0171/0396/6ec78.png)
![[AB]=K[A][B]](/tpl/images/0171/0396/ad806.png)
![Rate=KK[A][B][B]](/tpl/images/0171/0396/3919b.png)
![Rate=K'[A][B]^2](/tpl/images/0171/0396/8db87.png)


