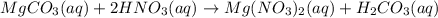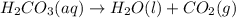Chemistry, 04.08.2019 16:30 aharvitt0417
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid form. carbonic acid then breaks down into water and carbon dioxide. what two types of reactions take place in this process?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1


Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid...
Questions



Chemistry, 12.02.2021 23:10


History, 12.02.2021 23:10


Mathematics, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10



Business, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10

Mathematics, 12.02.2021 23:10

Chemistry, 12.02.2021 23:10


Mathematics, 12.02.2021 23:10

SAT, 12.02.2021 23:10

English, 12.02.2021 23:10

History, 12.02.2021 23:10