
Chemistry, 04.08.2019 16:00 NeonPlaySword
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm o2=32 g/mol; mm fe2o3=159.70 g/mol) if 63.98 g of oxygen gas is completely consumed, how many moles of iron (iii) oxide are formed? a. 1.333 mol b. 3071 mol c. 2.999 mol d. 6812 mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
One of the reactions for rusting iron is as follows: 4fe + 3o2 → 2fe2o3 (mm fe: 55.85 g/mol; mm...
Questions

History, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40



English, 01.03.2021 19:40


Computers and Technology, 01.03.2021 19:40

Social Studies, 01.03.2021 19:40



Mathematics, 01.03.2021 19:40



History, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40

Mathematics, 01.03.2021 19:40

Biology, 01.03.2021 19:40

English, 01.03.2021 19:40


Mathematics, 01.03.2021 19:40

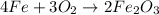
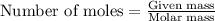
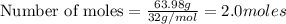
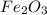 .
.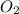 produces 2 moles of
produces 2 moles of 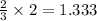 moles of
moles of 

