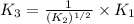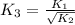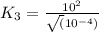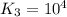Chemistry, 02.08.2019 06:40 kenldykido2300
For the hypothetical reactions 1 and 2, k1 = 102 and k2 = 10–4. 1. a2(g) + b2(g) 2ab(g) 2. 2a2(g) + c2(g) 2a2c(g) 3. a2c(g) + b2(g) 2ab(g) + (1/2)c2(g) what is the value for k for reaction 3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3


Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

You know the right answer?
For the hypothetical reactions 1 and 2, k1 = 102 and k2 = 10–4. 1. a2(g) + b2(g) 2ab(g) 2. 2a2(g) +...
Questions


Mathematics, 26.07.2019 03:30


Physics, 26.07.2019 03:30




Business, 26.07.2019 03:30

History, 26.07.2019 03:30

Social Studies, 26.07.2019 03:30





History, 26.07.2019 03:30




Health, 26.07.2019 03:30

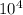
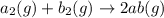 ;
; 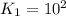
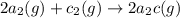 ;
; 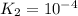
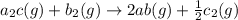
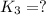
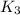 for the final reaction.
for the final reaction.