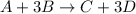Chemistry, 02.08.2019 00:30 callmedarthvadorplz
Look at the following hypothetical reaction: a+3b--> c+3d assume that the equation is balanced. in which of the following reactant mole ratios will reactant b be the limiting reagent? a) a: 4b b) a: 5b c) 2a: 7b d) a: b

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
Look at the following hypothetical reaction: a+3b--> c+3d assume that the equation is balanced....
Questions



Physics, 25.08.2019 08:50


Biology, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50



Mathematics, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

Physics, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50



English, 25.08.2019 08:50


Computers and Technology, 25.08.2019 08:50

Mathematics, 25.08.2019 08:50

English, 25.08.2019 08:50