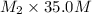Chemistry, 01.08.2019 02:10 nxusasmangaliso1191
If you have 25.0 ml of a 6.50m naoh solution and you add 10.0 ml of water to it, what is the new concentration?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
If you have 25.0 ml of a 6.50m naoh solution and you add 10.0 ml of water to it, what is the new con...
Questions



Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00


Mathematics, 08.07.2019 12:00

History, 08.07.2019 12:00


Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

History, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

Health, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00


Geography, 08.07.2019 12:00

Business, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

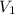 = 25.0 ml,
= 25.0 ml, 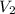 = (10.0 ml + 25.0 ml) = 35.0 ml
= (10.0 ml + 25.0 ml) = 35.0 ml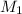 = 6.50 M,
= 6.50 M, 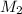
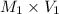 =
= 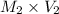
 =
=