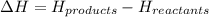Chemistry, 30.07.2019 09:20 lilyrockstarmag
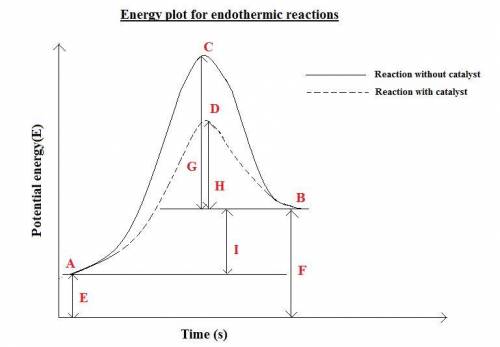
How is enthalpy used to predict whether a reaction is endothermic or exothermic? when the enthalpy of the reactants is higher than the enthalpy of the products, the reaction is endothermic. when the enthalpy of the products is higher than the enthalpy of the reactants, the reaction is exothermic. when the enthalpy change of the reaction is positive, the reaction is exothermic. when the enthalpy change of the reaction is positive, the reaction is endothermic.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
How is enthalpy used to predict whether a reaction is endothermic or exothermic? when the enthalpy...
Questions

Computers and Technology, 11.05.2021 01:00


Biology, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00



Mathematics, 11.05.2021 01:00



Arts, 11.05.2021 01:00





Mathematics, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00

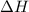 for the reaction comes out to be negative.
for the reaction comes out to be negative.