
Chemistry, 29.07.2019 19:00 elitehairnerd1964
Which of the following best explains the polarity of water (h2o)? a. protons are more attracted to the hydrogen atoms, so the hydrogen atoms become positively charged. b. since the oxygen atom has more electrons than the hydrogen atoms, it is negatively charged. c. electrons are more attracted to the oxygen atom, so there is a partial negative charge on the oxygen atom. d. since there are two positive hydrogen atoms and only one negative oxygen atom, the water molecule is positively charged.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Which of the following best explains the polarity of water (h2o)? a. protons are more a...
Questions




English, 08.11.2021 14:00

SAT, 08.11.2021 14:00




History, 08.11.2021 14:00

Mathematics, 08.11.2021 14:00

Mathematics, 08.11.2021 14:00


Mathematics, 08.11.2021 14:00


Mathematics, 08.11.2021 14:00

Chemistry, 08.11.2021 14:00




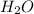 . This shows that there are two hydrogen atoms and one oxygen atom.
. This shows that there are two hydrogen atoms and one oxygen atom. 

