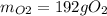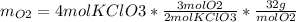The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info: mm o2 = 32 g/mol mm kcl = 74.55 g/mol mm kclo3 = 122.55 g/mol if 4.00 moles of kclo3 are totally consumed, how many grams of oxygen gas would be produced? 192 g 6.00 g 85.3 g 735 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info:...
Questions


English, 10.06.2020 04:57



History, 10.06.2020 04:57



History, 10.06.2020 04:57


Mathematics, 10.06.2020 04:57


English, 10.06.2020 04:57