
Chemistry, 11.10.2019 19:30 Cheflulu5727
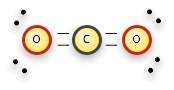
When carbon dioxide is formed, one carbon atom forms double covalent bonds with two oxygen atoms. carbon dioxide is considered a) less stable than the individual atoms because it is a gas. b) less stable than the individual atoms because it can be decomposed readily. c) more stable than individual atoms as now the outer energy levels of all atoms are filled. d) more stable than the individual atoms because together they form a more massive substance.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
When carbon dioxide is formed, one carbon atom forms double covalent bonds with two oxygen atoms. ca...
Questions

Business, 04.08.2019 00:20

Biology, 04.08.2019 00:20

Biology, 04.08.2019 00:20

History, 04.08.2019 00:20


Social Studies, 04.08.2019 00:20


Business, 04.08.2019 00:20

History, 04.08.2019 00:20


History, 04.08.2019 00:20

History, 04.08.2019 00:20




Business, 04.08.2019 00:20

Biology, 04.08.2019 00:20

Biology, 04.08.2019 00:20

Social Studies, 04.08.2019 00:20

Mathematics, 04.08.2019 00:20



