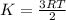Decreasing the temperature of a reaction decreases the reaction rate because a) decreasing the temperature also decreases the pressure. b) decreasing the temperature may cause a reactant to solidify. c) decreasing the temperature decreases the concentration of reactants. d) decreasing the temperature lowers the average kinetic energy of the reactants.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Decreasing the temperature of a reaction decreases the reaction rate because a) decreasing the tempe...
Questions


History, 12.03.2021 09:20

Mathematics, 12.03.2021 09:20


English, 12.03.2021 09:20


Chemistry, 12.03.2021 09:20

Biology, 12.03.2021 09:20

Mathematics, 12.03.2021 09:20


Mathematics, 12.03.2021 09:20

English, 12.03.2021 09:20



Mathematics, 12.03.2021 09:20

Mathematics, 12.03.2021 09:20



Mathematics, 12.03.2021 09:20

Mathematics, 12.03.2021 09:20