Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 23.06.2019 13:30
1. what is boyle’s law? • state the definition of the law in words. • what are the assumptions of boyle’s law? • write at least one mathematical equation that represents the law. • what can be calculated with boyle’s law? • using a gas-filled balloon as an example, describe what is happening to the gas molecules inside the balloon before and after you squeeze it.
Answers: 2
You know the right answer?
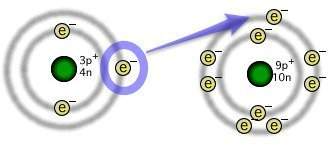
The illustration depicts the formation of an ionic chemical bond between lithium and fluorine atoms....
Questions

English, 01.08.2021 14:00

English, 01.08.2021 14:00



Mathematics, 01.08.2021 14:00




Biology, 01.08.2021 14:00

Mathematics, 01.08.2021 14:00

English, 01.08.2021 14:00

Mathematics, 01.08.2021 14:00

Biology, 01.08.2021 14:00

Mathematics, 01.08.2021 14:00

Mathematics, 01.08.2021 14:00

Biology, 01.08.2021 14:00

Mathematics, 01.08.2021 14:00

Biology, 01.08.2021 14:00


Mathematics, 01.08.2021 14:00