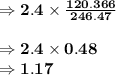Chemistry, 26.07.2019 12:00 Ilikepandas2019
Determine the quantity of pure mgso4 in 2.4 g of mgso 4 ⋅ 7h 2 o mgso4⋅7h2o

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Determine the quantity of pure mgso4 in 2.4 g of mgso 4 ⋅ 7h 2 o mgso4⋅7h2o...
Questions


Mathematics, 20.09.2020 14:01

English, 20.09.2020 14:01





Mathematics, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01

English, 20.09.2020 14:01




Computers and Technology, 20.09.2020 14:01


Biology, 20.09.2020 14:01


Physics, 20.09.2020 14:01

Health, 20.09.2020 14:01

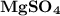 in 2.4 g of
in 2.4 g of 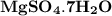 is 1.17 g.
is 1.17 g.