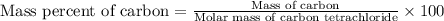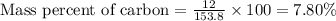Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Calculate the mass percent of carbon (c) in carbon tetrachloride, (ccl4), if the molar mass of carbo...
Questions


Social Studies, 05.05.2020 01:43


Mathematics, 05.05.2020 01:43

Biology, 05.05.2020 01:43

Mathematics, 05.05.2020 01:43








History, 05.05.2020 01:43

Mathematics, 05.05.2020 01:43




Social Studies, 05.05.2020 01:43

Chemistry, 05.05.2020 01:43

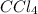 , there are 1 carbon atoms and 4 chlorine atoms.
, there are 1 carbon atoms and 4 chlorine atoms.