
Chemistry, 25.07.2019 02:00 JocelynC7237
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentration of oh− ions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2


Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Suppose you titrate 80.0 ml of 2.00 m naoh with 20.0 ml of 4.00 m hcl. what is the final concentrati...
Questions

Physics, 24.05.2021 20:30


Mathematics, 24.05.2021 20:30



History, 24.05.2021 20:30


Chemistry, 24.05.2021 20:30


English, 24.05.2021 20:30

World Languages, 24.05.2021 20:30


Spanish, 24.05.2021 20:30


Health, 24.05.2021 20:30



Mathematics, 24.05.2021 20:30

History, 24.05.2021 20:30

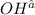 is 0.8 M.
is 0.8 M.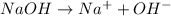
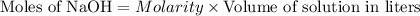
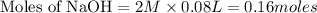
![[OH^-]](/tpl/images/0129/3993/b2910.png)


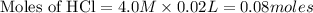
![[H^+]](/tpl/images/0129/3993/07acb.png)

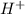 will react with 0.08 moles of
will react with 0.08 moles of 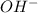 and (0.16-0.08)= 0.08 moles of
and (0.16-0.08)= 0.08 moles of ![[OH^]-=\frac{moles}{\text {Volume in L}}=\frac{0.08}{0.1}=0.8M](/tpl/images/0129/3993/83da4.png)


