
Chemistry, 24.07.2019 10:30 graymonky12
Balance the following equation. then determine the ratio for the products kcl and o2 generated during the decomposition of potassium chlorate. kclo3 kcl + o2 1: 1 2: 2 4: 3 2: 3 3: 2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Balance the following equation. then determine the ratio for the products kcl and o2 generated durin...
Questions


Mathematics, 13.07.2019 04:00


English, 13.07.2019 04:00


Biology, 13.07.2019 04:00



Biology, 13.07.2019 04:00

Mathematics, 13.07.2019 04:00






English, 13.07.2019 04:00

English, 13.07.2019 04:00


Mathematics, 13.07.2019 04:00

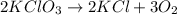
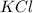 and 3 moles of
and 3 moles of 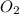 .
.


