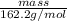Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
How many grams of iron (iii) chloride must decompose to produce 78.4 milliliters of iron metal, if t...
Questions

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

English, 15.09.2020 20:01

English, 15.09.2020 20:01

Chemistry, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

English, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

History, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Mathematics, 15.09.2020 20:01

Computers and Technology, 15.09.2020 20:01

History, 15.09.2020 20:01


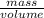
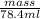
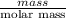
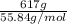
 : Fe is 2:2.
: Fe is 2:2.