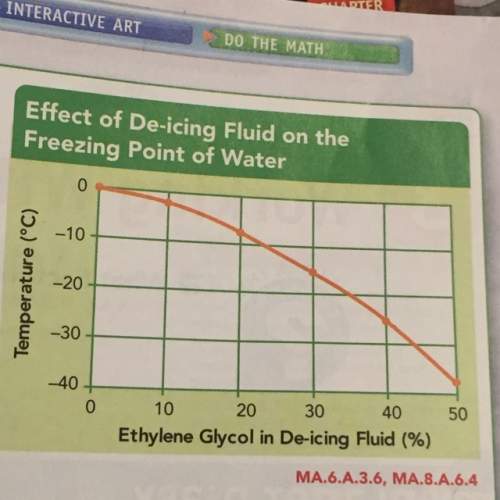
How is the percent of ethylene glycol in de-icing fluid related to water’s freezing point ?
...

Chemistry, 16.09.2019 04:30 stephliu721
How is the percent of ethylene glycol in de-icing fluid related to water’s freezing point ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
Questions

Mathematics, 16.11.2020 23:40



Medicine, 16.11.2020 23:40

Chemistry, 16.11.2020 23:40


Arts, 16.11.2020 23:40

English, 16.11.2020 23:40

Mathematics, 16.11.2020 23:40




Mathematics, 16.11.2020 23:40


Mathematics, 16.11.2020 23:40


English, 16.11.2020 23:40

Mathematics, 16.11.2020 23:40

Mathematics, 16.11.2020 23:40

English, 16.11.2020 23:40



