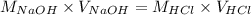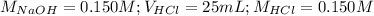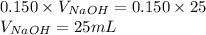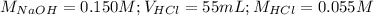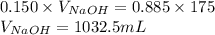Chemistry, 23.07.2019 20:10 mrylenastewart
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric acid. the neutralization reaction is: naoh(aq) + hcl(aq) → h2o(l) + nacl(aq) 25 ml of a 0.150 m hcl solution 55 ml of a 0.055 m hcl solution 175 ml of a 0.885 m hcl solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2



Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Determine the volume of 0.150 m naoh solution required to neutralize each sample of hydrochloric aci...
Questions

Mathematics, 22.04.2021 21:30

Mathematics, 22.04.2021 21:30


German, 22.04.2021 21:30




Mathematics, 22.04.2021 21:30


Advanced Placement (AP), 22.04.2021 21:30

Mathematics, 22.04.2021 21:30






Computers and Technology, 22.04.2021 21:30