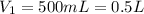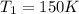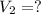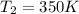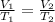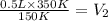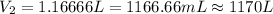Chemistry, 23.07.2019 10:00 ciarrap552
The temperature of a 500. ml sample of gas increases from 150. k to 350. k. what is the final volume of the sample of gas, if the pressure and moles in the container is kept constant? 0.0095 ml 110. ml 0.0470 ml 210. ml 1170 ml

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
The temperature of a 500. ml sample of gas increases from 150. k to 350. k. what is the final volume...
Questions






English, 11.10.2020 17:01





Chemistry, 11.10.2020 17:01


English, 11.10.2020 17:01

Mathematics, 11.10.2020 17:01

Mathematics, 11.10.2020 17:01

English, 11.10.2020 17:01


History, 11.10.2020 17:01

History, 11.10.2020 17:01

Mathematics, 11.10.2020 17:01