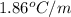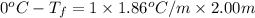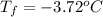Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

You know the right answer?
Calculate the freezing point of a 2.00 molal solution of the nonelectrolyte glucose. the freezing po...
Questions




Geography, 06.08.2019 02:30

Physics, 06.08.2019 02:30








Computers and Technology, 06.08.2019 02:30

Geography, 06.08.2019 02:30


Medicine, 06.08.2019 02:30




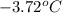
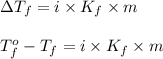
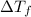 = change in freezing point
= change in freezing point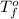 = temperature of pure water =
= temperature of pure water = 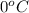
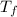 = temperature of solution = ?
= temperature of solution = ?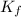 = freezing point constant =
= freezing point constant =