
Chemistry, 22.07.2019 18:50 haileysmile2006
The specific heat of copper is 0.385 j/(g · °c). if 34.2 g of copper, initially at 24.0°c, absorbs 4.689 kj, what will be the final temperature of the copper?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1

Chemistry, 23.06.2019 18:00
Astudent measured the ph for four solutions which ph indicated the lowest hydronium ion or concentration
Answers: 1
You know the right answer?
The specific heat of copper is 0.385 j/(g · °c). if 34.2 g of copper, initially at 24.0°c, absorbs 4...
Questions


Health, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00




Biology, 01.10.2019 22:00


Business, 01.10.2019 22:00






Mathematics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00

Mathematics, 01.10.2019 22:00

Biology, 01.10.2019 22:00


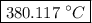
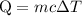 …… (1)
…… (1) is the change in temperature of copper.
is the change in temperature of copper.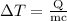 …… (2)
…… (2)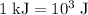
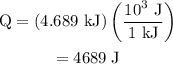
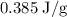 .
.
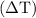 can be calculated as follows:
can be calculated as follows: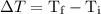 …… (3)
…… (3)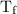 is the final temperature.
is the final temperature.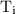 is the initial temperature.
is the initial temperature.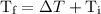 …… (4)
…… (4)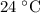 .
.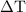 is
is 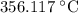
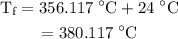
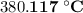 .
.


