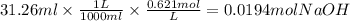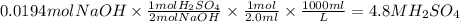Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
The molarity of sulfuric acid in a fully charged car battery is 5.2 m. when fully discharged the mol...
Questions


Mathematics, 01.09.2021 21:50

English, 01.09.2021 21:50


Mathematics, 01.09.2021 21:50

Biology, 01.09.2021 21:50

Social Studies, 01.09.2021 21:50

History, 01.09.2021 21:50

Mathematics, 01.09.2021 21:50

English, 01.09.2021 21:50

Business, 01.09.2021 21:50

Mathematics, 01.09.2021 21:50

Social Studies, 01.09.2021 21:50

English, 01.09.2021 21:50

Mathematics, 01.09.2021 21:50



English, 01.09.2021 21:50


Geography, 01.09.2021 21:50

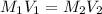 , where
, where 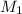 is the molarity of the acid sulfuric acid,
is the molarity of the acid sulfuric acid, 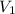 the volume of acid,
the volume of acid, 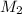 is the molarity of the base sodium hydroxide and
is the molarity of the base sodium hydroxide and 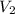 is the volume of the base.
is the volume of the base.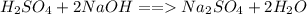 .
. 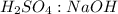 is 1:2. Which means 1 mol
is 1:2. Which means 1 mol 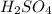 react with 2 mol
react with 2 mol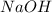 . Using the volume and molarity of
. Using the volume and molarity of